Professor Myung Jae-ha of Incheon National University presents a paper on nanoparticle elution mechanism and strategy for perovskite oxides
- 글번호
- 385335
- 작성일
- 2024-04-02
- 수정일
- 2024-04-02
- 작성자
- 홍보팀 (032-835-9490)
- 조회수
- 318

Photographs of Professor Myung Jae-ha's lab researcher
A research team led by Professor Myung Jae-ha of the Department of New Materials Engineering at Incheon National University (co-author: Kim Yo-han, Jeong Hyeon-kwon, PhD student at Won Boram) published a paper on the elution phenomenon mechanism and control factors of perovskite oxide, and the latest strategies for maximizing elution nanoparticle growth.
The elution method covered in this study is a technology in which a catalyst metal is doped into an oxide support lattice and then grown in the form of fixed nanoparticles on the surface through a reduction process.
Because the eluted nanocatalyst has a socket structure fixed to the oxide support and exhibits a uniform particle distribution, it can maximize the active area where an electrochemical reaction occurs, and exhibits high stability with high-temperature aggregation and resistance to carbon deposition.
It was suggested that it can be used in various fields such as hydrogen fuel cells, water electrolysis cells, and reforming catalysts by understanding the characteristics of the eluted nanocatalyst.
This study analyzed the advantages and disadvantages by combining the recent research results of Professor Ha's team and the latest strategies to overcome growth limitations proposed by various research groups from various perspectives such as reaction kinetics, thermodynamics, and quantum mechanics.
However, in order to further understand the complex relationship between the properties and control factors of elution nanoparticles, it emphasized the need for a lot of research to fuse multi-faceted analysis experiments and theoretical modeling, and suggested in-depth what elution technology should proceed.
This study was conducted exclusively by Professor Myung Jae-ha of Incheon National University and was supported by the Korea Research Foundation-Excellent New Research Research and the Ministry of Trade, Industry and Energy-New and Renewable Energy Core Technology Development Project with the funding of the Ministry of Science and ICT, and was published in Nano-Micro Letters (IF=26.6), an international academic journal in the field of nano.
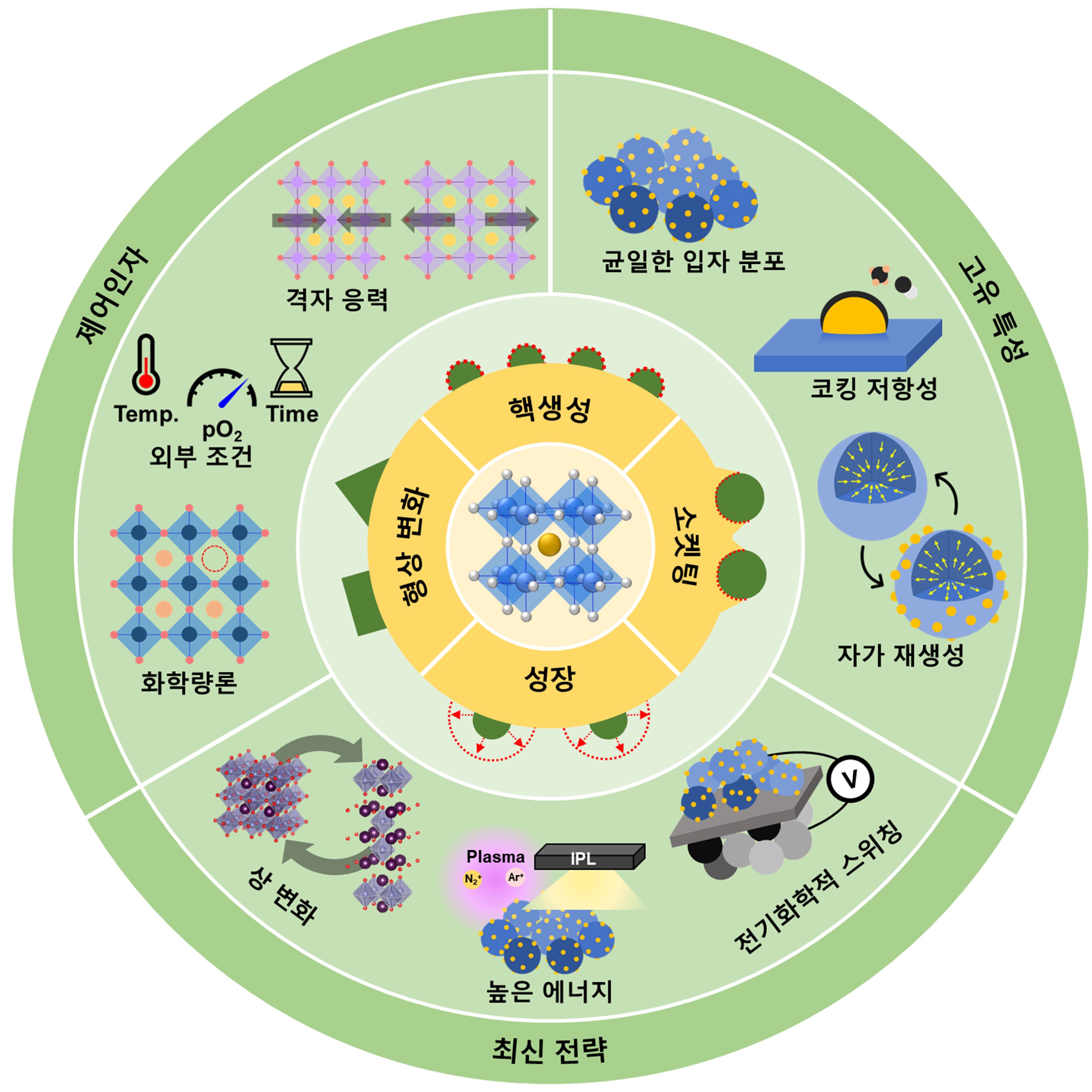
A schematic diagram of nanoparticle elution mechanism, control factors, intrinsic properties, and state-of-the-art strategies of perovskite oxide materials
[Picture Description]
The dissolution of nanoparticles from a perovskite oxide material is formed through a nucleation-socketing-growth process, and the shape of the dissolution particles changes under special conditions.
Typical control factors of elution phenomena include lattice stress, reduction temperature, reduction time, oxygen partial pressure (pO2), and stoichiometry.
Unlike conventional methods (physical deposition, impregnation), nanoparticles grown through elution have a uniform particle distribution, are resistant to carbon deposition in carbon-based fuels such as CH4 and CO, and can self-regenerate through repeated oxidation/reduction reactions.
As the latest strategy for maximizing nucleation and growth of eluted nanoparticles, there is a method of utilizing (1) phase change, (2) high energy such as plasma, and light laser, and (3) electrochemical potential.
- 첨부파일
-
- A schematic diagram of nanoparticle elution mechanism, control factors, intrinsic properties, and state-of-the-art strategies of perovskite oxide materials.jpg
- Professor Myung Jae-ha of Incheon National University presents a paper on nanoparticle elution mechanism and strategy for perovskite oxides.jpg